报告
欧盟医疗人工智能研究和应用现状
摘要
欧洲一直是科学研究的前沿地区之一,近年来人工智能在欧洲也得到了迅速发展。随着人口老龄化和人力资源短缺等一系列社会问题的凸显,欧洲国家和地区也希望通过人工智能技术解决在医疗行业和健康领域出现的问题。本文首先对欧盟在医疗人工智能方面制定的政策进行分析,总结欧盟医疗人工智能战略的具体内容和实施框架,说明人工智能技术在医疗行业的重要性;其次,分析当前欧盟在医疗人工智能产品的准入标准以及当前主要的临床应用产品,说明人工智能技术在医疗行业的产业化情况;再次,分析欧盟对医疗人工智能在伦理方面关注的热点,说明医疗人工智能产品应该遵守的法律和伦理原则;最后,结合欧洲医学人工智能大会历年论文发表情况,总结当前欧洲医学人工智能研究要点及前沿。
作者
黄智生 ,北京智通智慧科技有限公司,首席科学家,主要研究方向为知识图谱、人工智能等领域开发及应用。
王海渊 ,北京智通智慧科技有限公司,总工程师,主要研究方向为知识图谱、人工智能等领域开发及应用。
胡青 ,北京智通智慧科技有限公司,研究员,主要研究方向为医学人工智能。
张雅涵 ,北京智通智慧科技有限公司,研究员,主要研究方向为人工智能的知识产权布局及保护。
Huang Zhisheng
Wang Haiyuan
Hu Qing
Zhang Yahan
检索正文关键字
报告目录
-
一 欧盟医疗人工智能政策研究
- (一)欧洲人工智能(Artificial Intelligence for Europe)
- 1.提高技术和工业能力、促进人工智能在经济中的应用
- 2.迎接人工智能带来的社会经济变革
- (二)人工智能协调计划(Coordinated Plan on Artificial Intelligence)
- (三)医疗在人工智能政策中的重要性
- (一)欧洲人工智能(Artificial Intelligence for Europe)
-
二 欧盟医疗人工智能产品标准及应用研究
- (一)人工智能产品的定义
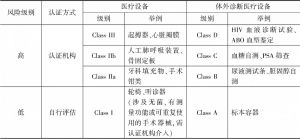
- (二)欧盟医疗人工智能产品准入标准
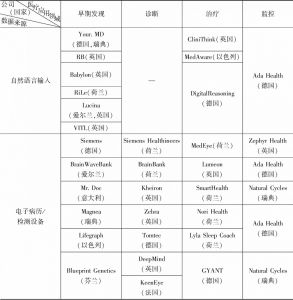
- (三)临床应用产品
- (1)早期发现与分类
- (2)诊断
- (3)治疗管理
- (4)监测/结果改进
-
三 欧盟医疗人工智能立法及伦理分析研究
- (一)医疗人工智能中存在的立法及伦理问题
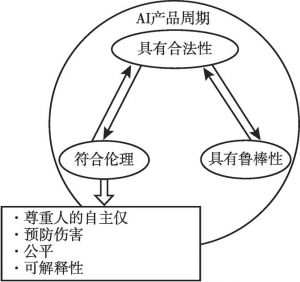
- (二)可信赖的人工智能(Trustworthy AI)
- (三)《通用数据保护条例》(General Data Protection Regulation)
-
四 欧盟医疗人工智能前沿技术展望研究
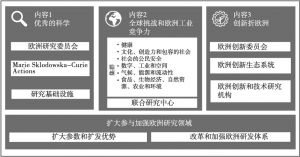
- (一)地平线欧洲(Horizon Europe)计划
- (二)欧盟医疗人工智能应用技术展望
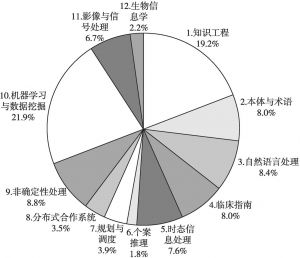
- 1.机器学习与数据挖掘
- 2.知识工程
- 3.自然语言处理
- 4.非确定性处理
- 5.本体与术语
相关文献
查看更多>>>